A novel therapeutic giving cancer patients new hope.
We are focused on rapidly advancing our lead therapeutic candidate, misetionamide (GP-2250), for the treatment of multiple cancer types including ovarian and pancreatic cancers.
Our Science
Disrupting cancer cell metabolism.
Our unique and novel mechanism of action selectively disrupts the energy metabolism of cancer cells leading to cancer cell death as well as impacting nuclear factor-κB (“NFκB”) which effects cancer cells’ ability for protein synthesis and DNA transcription thereby restricting cancer cell growth and proliferation.
Our PIPELINE
Focused on Addressing Unmet Needs for Patients with Cancer.
Ovarian
Cancer
Pancreatic
Cancer
Additional
Targets
Melanoma, Squamous Cell, Breast, Colorectal
Truly novel.
Truly important.
We are on a mission to improve therapeutic outcomes for cancer patients with misetionamide, a tumor-cell selective agent with a truly novel mechanism of action.
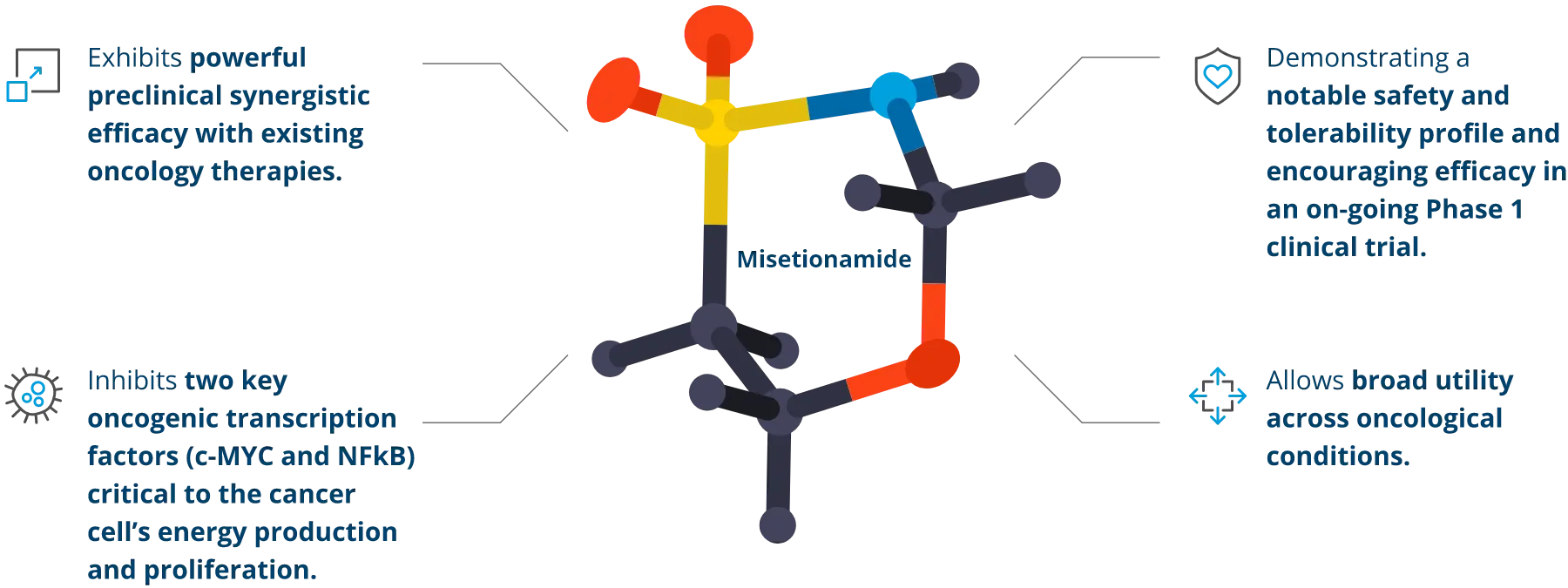
Focused on what matters most.
In addition to demonstrating direct anti-cancer activity, misetionamide has shown pro-inflammatory cytokine suppression in preclinical studies.
Misetionamide’s inhibition of c-MYC potentially offers the opportunity of enhancing immunotherapy as well.
We have chosen to focus on the treatment of ovarian and pancreatic cancers as our initial indications, two conditions with a clear unmet medical need. Early clinical experience is promising.