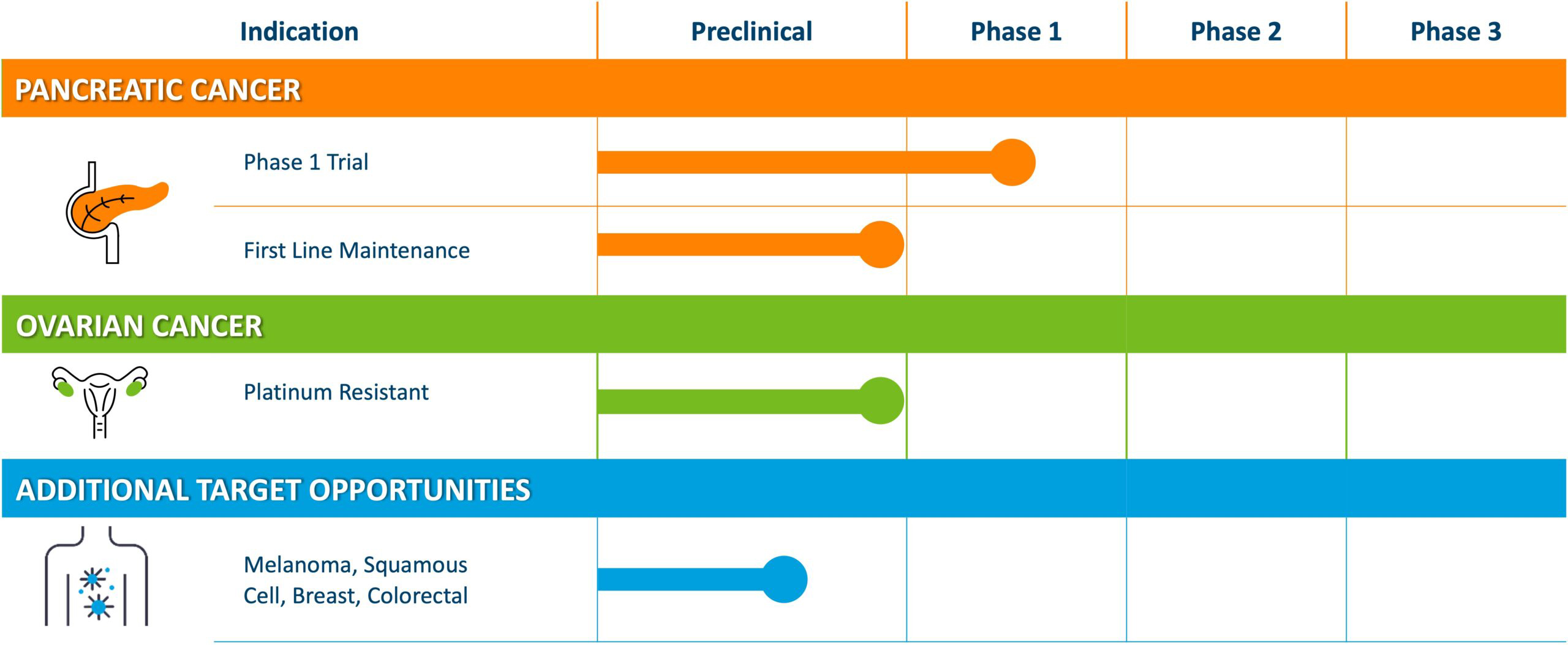
Misetionamide Pipeline
Our pipeline includes an active phase 1 dose escalation trial of misetionamide in combination with gemcitabine for pancreatic cancer, and a trial in ovarian cancer in combination with bevacizumab and PLD to initiate in 2025.

PROMISING PIPELINE
Broad applicability & potential.
Misetionamide, our lead asset, is a broadly active small molecule with a unique mechanism of action that inhibits two oncogenic transcription factors, c-MYC and NFκB. Acting through c-MYC, misetionamide selectively disrupts the energy metabolism of cancer cells leading to cancer cell death. Through NFκB inhibition, misetionamide inhibits cancer cells’ ability to proliferate and survive.
This powerful anti-cancer, dual-target mechanism of action makes misetionamide an attractive tumor cell selective agent across many cancers.

Participate
Become a clinical investigator or patient participant.
Are you a clinician or patient who is interested in learning about or participating in our active studies?
Science
Discover our novel mechanism of action.
Misetionamide’s mechanism of action inhibits two oncogenic transcription factors, c-MYC and NFκB.