About Misetionamide
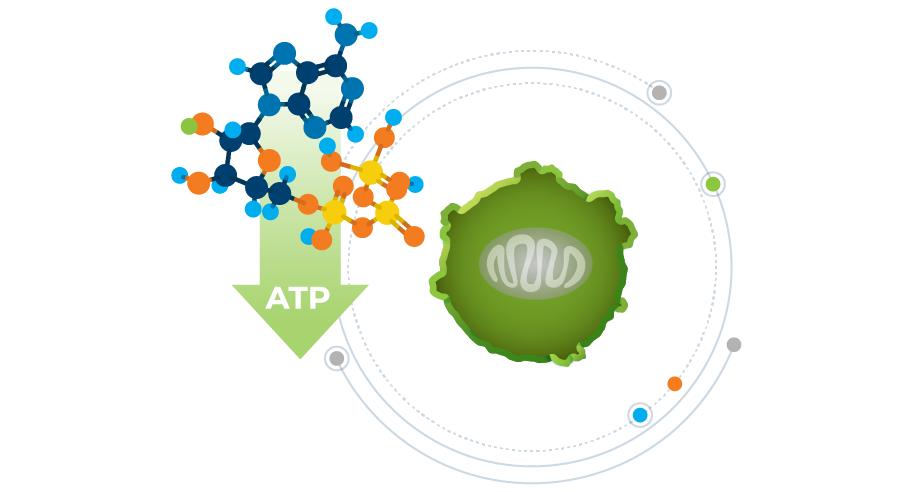
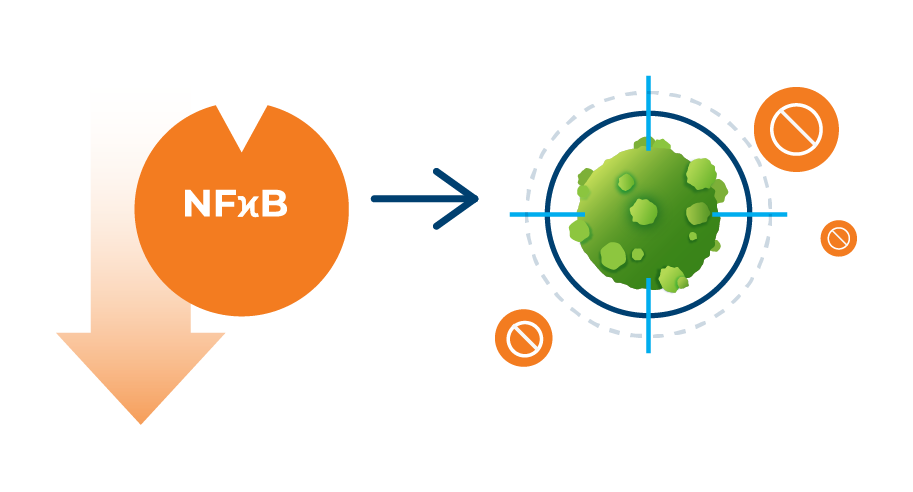
Misetionamide is a broadly active small molecule with a unique mechanism of action that inhibits two oncogenic transcription factors, c-MYC and NFκB. Acting through c-MYC, misetionamide selectively disrupts the energy metabolism of cancer cells leading to cancer cell death. Through NFκB inhibition, misetionamide inhibits cancer cells’ ability to proliferate and survive. This mechanism makes misetionamide an attractive tumor cell selective agent.

Differentiators
What Sets Misetionamide Apart?
Broad oncology applications.
Misetionamide is a tumor-type agnostic agent that delivers broad utility across cancer etiologies.
Cancer cell selective.
Because it targets the energy metabolism of cancer cells specifically, misetionamide is highly tumor cell selective.
Promising Data.
Extensive preclinical studies demonstrated both an excellent safety profile and anti-tumor activity.
The on-going clinical trial shows that misetionamide has a safe and tolerable profile with encouraging efficacy.
Synergistic activity.
Misetionamide has demonstrated powerful synergies with many commonly utilized anti-cancer agents.
Truly unique MOA.
Misetionamide leverages a unique and proprietary mechanism of action.
Interested in learning more?
Mechanism of Action
Novel, proprietary, transformative.
Misetionamide is a tumor cell selective and broadly active small molecule with a unique dual mechanism of action of selectively disrupting the energy metabolism of cancer cells leading to cancer cell death as well as impacting nuclear factor-κB (“NFκB”) which effects cancer cells’ ability for protein synthesis and DNA transcription thereby restricting cancer cell growth and proliferation.
Intellectual Property
Robust, multi-layered protection. Portfolio of 100+ issued patents.
- Composition of Matter (NCE & Formulations) Granted US & EU (2038 including patent extensions)
- Manufacturing Process (2035-2036)
- Method of Treatment (2037 – 2043)
- Mechanism of Action (2040)
- Additional Compounds (up to 2042)
Pipeline
Broad applicability, intentional focus.
In addition to demonstrating direct anti-cancer activity, misetionamide has shown pro-inflammatory cytokine suppression in preclinical studies.
Misetionamide’s inhibition of MYC potentially offers the opportunity of enhancing immunotherapy as well.
We have chosen to focus on the treatment of ovarian and pancreatic cancers as our initial indications, two conditions with a clear unmet medical need. Early clinical experience is promising.